AMPHIPOLS: Properties of membrane protein/amphipol complexes
When properly prepared, MP/A8-35 complexes are essentially monodisperse (Picard et al. (2006), Gohon et al. (2008), Zoonens et al. (2007)). It is however difficult to totally avoid the presence of minor fractions of small oligomers, which can seriously complicate, in particular, radiation scattering experiments (Gohon et al. (2008)). Among the factors that can lead to aggregation are the use of too little APol at the trapping step (Zoonens et al. (2007)), lowering the pH at or below neutrality (Gohon et al. (2008)), the presence of calcium ions (Picard et al. (2006)), drifts from the nominal composition of the polymer (Picard et al. (2006), Gohon et al. (2008)), and removal of the extra, free polymer that co-exists with MP/APol complexes at the end of a trapping experiment (Zoonens et al. (2007)). The latter phenomenon, which is reversible, is probably due to the poor dispersive power of APols: the presence of free polymer favors the formation of MP monomers, while its removal shifts the equilibrium towards protein auto-association. Although few proteins have been tested yet, it seems that freezing MP/APol complexes is not detrimental, while lyophilizing them can be (ref. Gohon et al. (2008), and unpublished data).
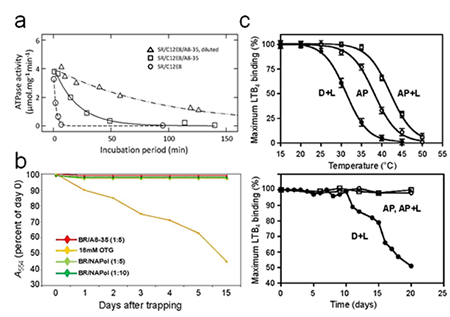
Complexation by A8-35 will, as a rule, stabilize biochemically a MP as compared to detergent solutions (Fig.4; reviewed in ref. Popot et al. (2011)). The underlying mechanisms are multiple (cf. Table 1 in Applications; for discussions, see ref. Popot et al. (2011)).
The functionality of APol-trapped MPs is generally preserved, as is their ability to bind ligands (reviewed in ref. Popot et al. (2011)). However, MPs whose functional cycle involves large rearrangements of the surface of their transmembrane region, like the sarcoplasmic calcium ATPase, may see their activity reversibly slowed down or blocked (Sharma et al. (2012)), presumably because the adsorbed polymer damps such transconformations (for a discussion, see refs. Popot et al. (2003, 2011), Picard et al. (2006)). The latter phenomenon ('Gulliver effect') probably contributes to slowing down MP denaturation (Popot et al. ( 2011), Picard et al. (2006)).